Transplant Surgery
Medical team:
Senior Consultant University Professor Dr. Rupert Oberhuber, PhD, FEBS (Teamleader)
Specialist Dr. Ruben Bellotti
Senior Consultant Privatdozent Dr. Benno Cardini, FEBS
Senior Consultant Privatdozentin Dr. Katrin Kienzl-Wagner
Senior Consultant Associate Professor Privatdozent Dr. Manuel Maglione, MBA, FEBS
Senior Consultant Privatdozent Dr. Herbert Maier
Senior Consultant Privatdozent Dr. Christian Margreiter, FEBS
Senior Consultant Privatdozentin Dr. Franka Messner-Rooprai, PhD, FEBS
Specialist Dr. Andras Meszaros, PhD
Senior Consultant Privatdozent Dr. Thomas Resch, PhD, FEBS
Senior Consultant Dr. Stefan Scheidl, FEBS
University Professor Dr. Stefan Schneeberger, MBA, FEBS
Consultation hours:
Tue 08:00 a.m. – 1:00 p.m.
Tel.: +43 512 504 22603
lki.ch.transplant-office@tirol-kliniken.at
A-6020 Innsbruck, Anichstraße 35, House 8, ground floor, outpatient clinic
24/7 Transplant coordination
Tel. +43 512 504 22603, Raum 8-G12-041
lki.ch.transplant-office@tirol-kliniken.at bzw. sandra.baher@tirol-kliniken.at
Sandra Baher
Elias Loewit
Julia Mader
Anna Scheiber
Kristina Strobl
Stephanie Wörgötter
A-6020 Innsbruck, Anichstraße 35, House 8, 12th floor, on the left side of the corridor after exiting the elevator

The following organ transplantations are performed at the Clinical Division of Visceral, Transplantation, and Thoracic Surgery:
Kidney transplantation
Liver transplantation
Pancreas transplantation
Small bowel and multivisceral transplantation
Hand transplantation
Referral information for healthcare providers treating patients with liver metastases from colorectal cancer (mCRC)
Within a clinical observational study (EC number: 1050/2019), we offer liver transplantation as a treatment option for patients with liver metastases from colorectal cancer. According to current knowledge, this approach can provide a significant survival benefit for selected patients compared to the previous standard therapy.
To avoid prolonged waiting times, living donor liver transplantation is an option that can be assessed on an individual basis. If you are treating a patient who may benefit from this, we kindly ask you to contact us.
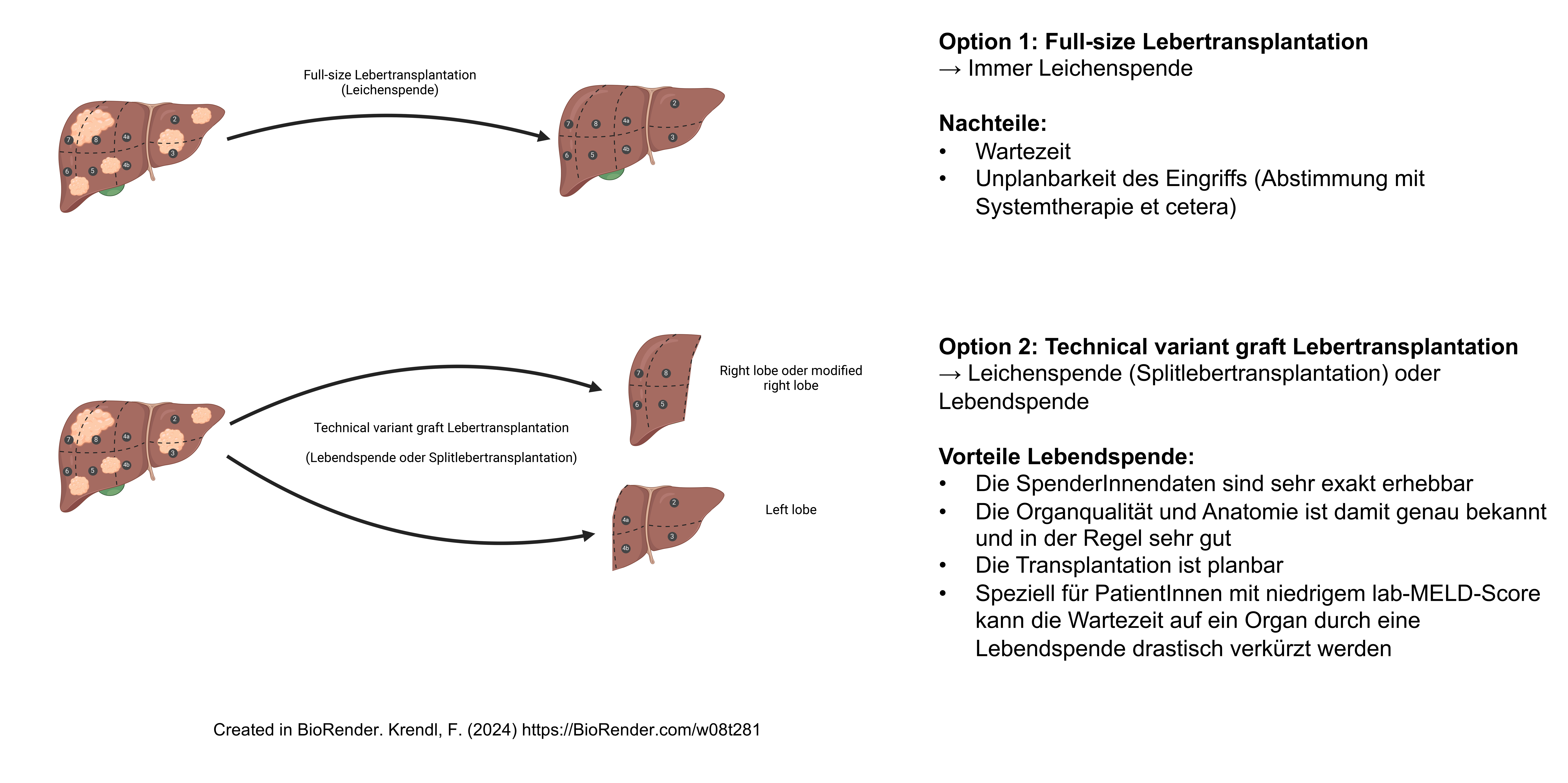
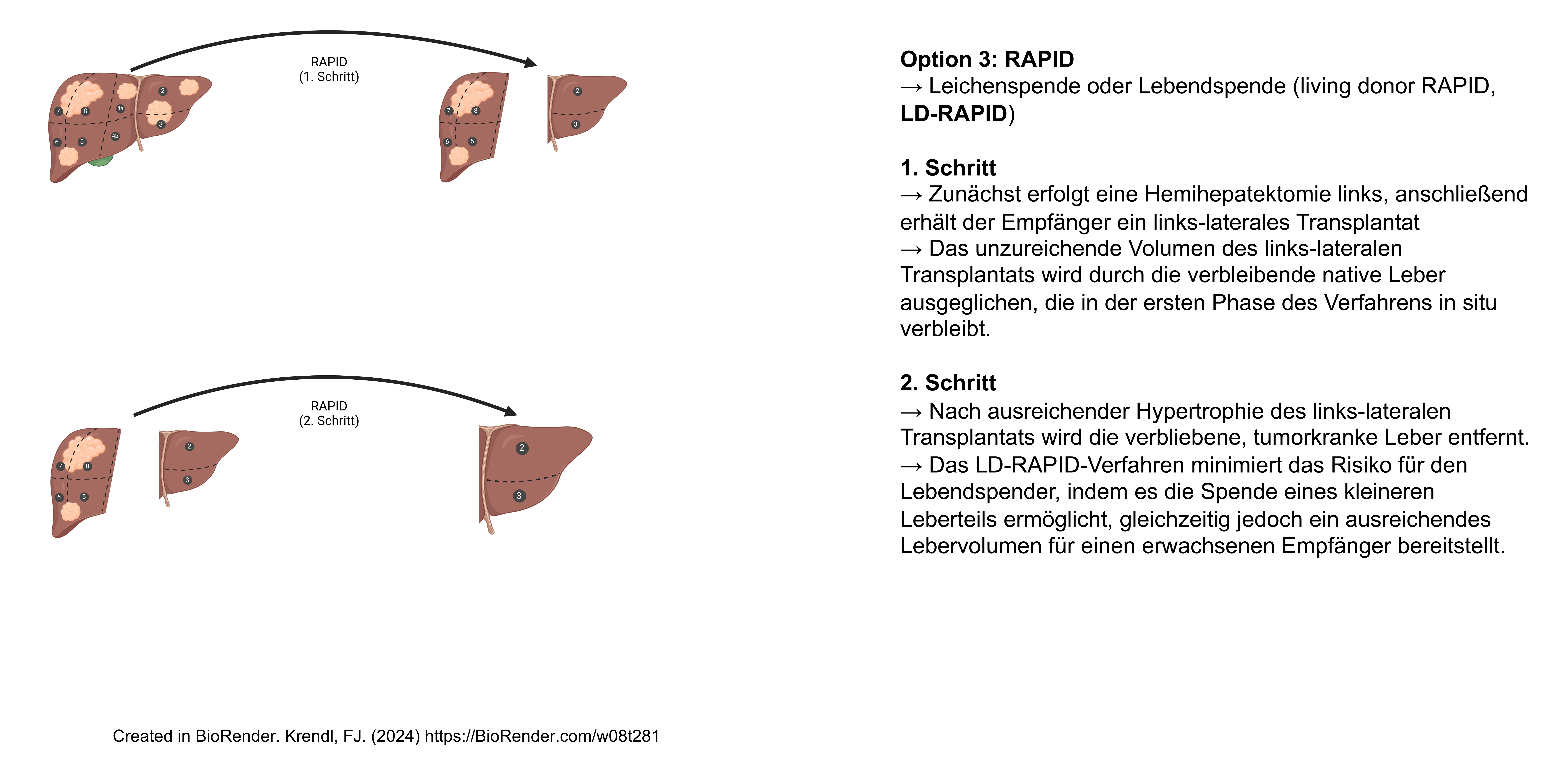
Criteria for being considered eligible for liver transplantation (LTx) in principle:
- Unresectable colorectal liver metastases
- No extrahepatic metastases
- BRAF V600Ewt
- Microsatellite‑stable tumor (mismatch repair proficient)
- Left‑sided primary tumor, histologically confirmed
Additional criteria that must be met prior to liver transplantation:
- R0 resection of the primary tumor according to oncologic criteria
- Stable disease (SD) or partial response (PR) of the liver metastases for at least 6 months under systemic therapy
- Preferably: CEA < 80 µg/L or a reduction of at least 50 % from baseline
- Relative contraindication: CEA > 80 µg/L with a decreasing trend
- Absolute contraindication: CEA > 80 µg/L with an increasing trend
- Preferably: metabolic tumor volume (MTV) ≤ 70 mL
- Preferably: fewer than four positive regional lymph nodes in the colon resection specimen (N2 = relative contraindication)
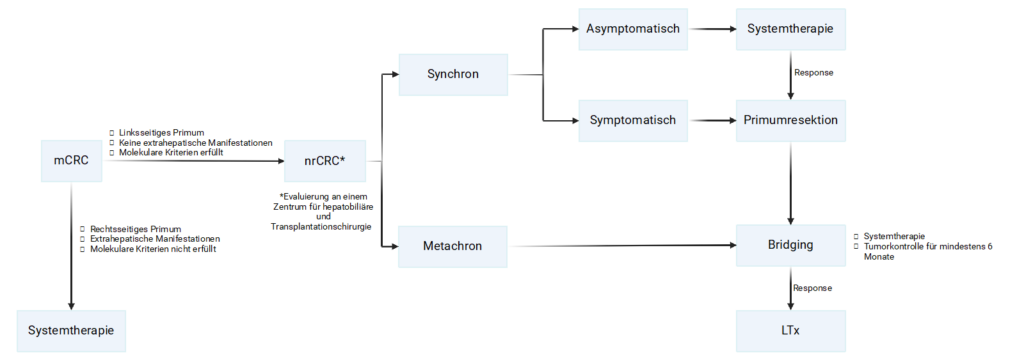
Therapy pathway to liver transplantation
In the figure below, the steps from initial diagnosis to liver transplantation are shown. Deviations from this pathway are possible but should be evaluated on a case‑by‑case basis. Contact for assessment of the criteria should ideally occur early after diagnosis so that liver transplantation can be considered as a treatment option.
Oncologic transplantation contact persons
University Professor Dr. Stefan Schneeberger, MBA, FEBS
University Professor Dr. Rupert Oberhuber, PhD, FEBS
Dr. Felix J. Krendl
Contact: lki.ch.transplant-office@tirol-kliniken.at
Further reading
- Bonney GK et al. Liver transplantation for non-resectable colorectal liver metastases: the International Hepato-Pancreato-Biliary Association consensus guidelines. Lancet Gastroenterol Hepatol. Nov 2021;6(11):933-946. Lancet Gastroenterol Hepatol. doi:10.1016/s2468-1253(21)00219-3
- Krendl FJ et al. Transplant oncology – Current indications and strategies to advance the field. JHEP Reports. 2024;6(2)doi:10.1016/j.jhepr.2023.100965
- Adam R et al. Liver transplantation plus chemotherapy versus chemotherapy alone in patients with permanently unresectable colorectal liver metastases (TransMet): results from a multicentre, open-label, prospective, randomised controlled trial. Lancet. Sep 21 2024;404(10458):1107-1118. doi:10.1016/s0140-6736(24)01595-2
- Dueland S et al. Long-Term Survival, Prognostic Factors, and Selection of Patients With Colorectal Cancer for Liver Transplant: A Nonrandomized Controlled Trial. JAMA Surg. Jul 26 2023:e232932. doi:10.1001/jamasurg.2023.2932
Kidney transplantation
The most common underlying diseases according to international statistics are: chronic glomerulonephritis, diabetic nephropathy, polycystic kidney disease, chronic pyelonephritis/interstitial nephritis, severe malformations of the kidney/urinary outflow tract, severe hypertension.
Less common underlying diseases include: Goodpasture syndrome, systemic lupus erythematosus, Wegener’s granulomatosis, Alport syndrome, pregnancy‑induced toxemia, hemolytic‑uremic syndrome, severe kidney damage caused by analgesics (analgesic nephropathy), drug‑induced chronic kidney disease.
Before establishing the indication for kidney transplantation, every existing progressive kidney disease must be evaluated on a case‑by‑case basis for its potential reversibility. This evaluation is carried out together with the transplant surgeon after comprehensive preliminary examinations and detailed discussions with the patient. The waiting time for a kidney transplant at our center is currently approximately two to six years. It should be emphasized that the allocation of suitable donor kidneys to recipients is strictly regulated by the organization “Eurotransplant” (based in Leiden, the Netherlands) according to tissue‑type similarity (HLA histocompatibility), using computer‑based matching once the respective blood‑tissue typing results of donor and recipient are available. The unfortunately long waiting time is explained by the increasing number of patients seeking kidney transplantation and the internationally well‑known general shortage of donor organs.
A favorable option in this situation is living‑donor kidney transplantation.
There is no definitive age limit for kidney transplantation. Provided the physical requirements are met (assessed through preliminary examinations), kidney transplantation can be performed from early childhood to advanced old age. It is worth highlighting the successful Eurotransplant Senior Program, which provides for the transplantation of kidneys from donors over 65 years of age to recipients also over 65 years old and has shown good long‑term outcomes. The goal of our kidney transplantation program is to ensure stable long‑term kidney function with good quality of life, the earliest possible return to work, and the realization of personal and family interests.
Living kidney donation
If, after careful consideration, extensive examinations, and consultations within the family or close emotional circle, a person willing to donate a kidney voluntarily is identified, this person will also undergo an intensive medical check‑up. The donor’s general health status, medical suitability as a kidney donor, and the anatomy and quality of the potential donor kidney are thoroughly evaluated. Donor and recipient must also undergo a detailed psychological counseling session. If all medical and psychological requirements are met, one kidney is removed from the donor—usually through a minimally invasive (“keyhole”) surgical procedure—and transplanted into the recipient (see surgical technique below).
One of the major advantages of living‑donor kidney transplantation is the ability to plan the timing of the operation. While a recipient on dialysis awaits a deceased‑donor organ, their health condition may often slowly deteriorate. Early living donation (possibly even as a pre‑emptive transplant before the recipient becomes dialysis‑dependent) can prevent this. At the time of transplantation, the recipient is therefore “healthier” and recovers more quickly from the procedure. Another advantage lies in the better organ quality, as the donor organ can be carefully selected. In addition, due to the ability to schedule the procedure, the time between organ removal and transplantation – during which the donor kidney must be cooled and stored (the so‑called “cold ischemia time”) – can be minimized. The result is significantly better outcomes compared with deceased‑donor transplantation, meaning longer patient survival and longer graft function. On average, the 5‑year graft survival rate (the percentage of kidneys that still show good function five years after transplantation) is approximately 90 %.
The procedure of kidney removal, as well as living with only one kidney afterward, is not entirely without risk for the donor. The operation itself carries the usual risks of surgical complications (e.g., postoperative bleeding or infections), although these are relatively low compared with other major abdominal surgeries. In addition, donors have a slightly increased risk – compared with the general population – of developing high blood pressure (arterial hypertension) or another cardiovascular disease within the next 10 to 20 years. This appears to affect all donors, but especially older ones. Approximately 30 % of donors over the age of 60 develop hypertension requiring treatment within 5 to 10 years. The risk of becoming dialysis‑dependent within 10 years after kidney donation is estimated at about 1 %. Although the overall risks for the donor are manageable, these figures underscore the importance of the donation being entirely voluntary. Furthermore, all donors should undergo regular follow‑up examinations with an internist after kidney removal. Following the usual inpatient stay of 5 – 10 days, the recovery period typically lasts 4 – 6 weeks.
After a thorough medical evaluation of a person willing to donate a kidney voluntarily—whether a family member or someone emotionally close—and after detailed medical assessment of kidney quality, clinical suitability, and anatomy, a donor kidney is transplanted into the recipient (see surgical technique below). The advantage of living‑donor kidney transplantation lies in the ability to plan the timing of the operation, the short waiting period, and especially the very short “cold ischemia time” (the time the kidney remains outside the bloodstream), which is typically longer for kidneys from unrelated deceased donors due to potential transport distances.
Surgical technique
The donor kidney is transplanted into the recipient through a curved incision in the right or left lower abdomen, but below the actual abdominal cavity. For the kidney to function normally in the recipient, its vessels must be connected to the recipient’s blood supply. The renal artery and renal vein are connected to the pelvic vessels, i.e., the recipient’s iliac artery and iliac vein. The ureter of the donor kidney is sewn into the bladder. To protect the suture, a stent (splint) is inserted during the operation. The stent is removed later by cystoscopy. In addition, at the end of the procedure a small drainage tube (Redon drain) is placed in the surgical area to drain any residual tissue fluid or blood to the outside. This drain is also removed after a few days. After the operation, regular ultrasound examinations and daily blood tests—especially kidney function parameters—are performed. To precisely adjust immunosuppression (see below), the blood concentrations of the corresponding medications must also be measured daily. This serves to continuously prevent possible rejection reactions. In some cases after the operation, if the kidney needs a bit of “start‑up time,” a few dialysis sessions may be necessary until the transplant achieves optimal blood‑filtration function and urine production. An acute rejection reaction is the body’s immune response against the transplanted foreign tissue. Through careful and frequent examinations (laboratory tests, ultrasound, kidney tissue sampling = biopsy), an acute rejection reaction can be detected quickly and almost always treated successfully: the so‑called cellular (i.e., T‑lymphocyte–mediated) rejection responds very well to high‑dose corticosteroid therapy. The less common “antibody‑mediated” rejection improves with plasma exchange (removal of antibodies), supplemented by medication administered intravenously to suppress lymphocytes. If the postoperative course is uncomplicated, the patient can be discharged in good general condition with stable transplant function after 1 to 3 weeks, with scheduled regular outpatient follow‑up visits at our center or, for patients living farther away, in close cooperation with their local centers. If any questions or problems arise, we are always reachable by phone.
Immunosuppression
The acceptance of the foreign kidney transplant by the recipient’s body is not a matter of course. Rather, the recipient’s organism would, from a biological standpoint, be ready to recognize the new tissue as foreign and reject it through an inflammatory reaction (rejection). To prevent this, a medical therapy suppressing the body’s immune system is administered starting at the time of transplantation; initially, this also includes corticosteroids, which can be reduced and ideally discontinued over the long term. A combination of medications is given that suppress the proliferation capacity of immune cells (lymphocytes) without completely destroying them, since they are still needed for other protective functions (against infections and tumors). A number of medications are suitable for preventing rejection: a combination of i) calcineurin inhibitors (tacrolimus or cyclosporine A), ii) a cell‑division inhibitor (mycophenolic acid, mTOR inhibitors, azathioprine), and iii) corticosteroids has proven effective. These medications are a long‑term maintenance therapy, but their dosage can be significantly reduced over time, allowing good quality of life and physical performance under this treatment. Additionally, to initiate immunosuppression, many patients receive an antibody during and shortly after the transplantation that reduces the number of immune cells in the recipient. An important task of the above‑mentioned regular outpatient follow‑up visits is dosage control and measurement of the drug levels in order to avoid over‑ or under‑dosage and potential side effects. Since there is considerable interest on the part of patients, physicians, and the pharmaceutical industry in optimizing and further developing this therapy, the approval of new immunosuppressive medications can indeed be expected in the coming years.
Side effects of immunosuppression
Because the immune system is artificially suppressed, an increased risk of infection is to be expected, especially during the initial phase after the transplantation. Patients are thoroughly informed and advised about this during pre‑transplant consultations and throughout their hospital stay. Discharge after transplantation is planned only once the patient can feel safe from an infectious‑disease standpoint. During the first two months after the transplant, a cautious approach to potential sources of infection is recommended: during this period, larger crowds, direct contact with infectious individuals, and heavy exposure to dust or dirt should be avoided. Over the following months, this risk decreases as the doses of the medications mentioned are reduced. Our particular concern is to maintain good personal contact with our patients; therefore, we place great importance on patients reaching out by phone should any questions arise or problems occur.
Results
At our center, more than 4,000 kidney transplants were performed between 1974 and 2016. The “durability” of kidney transplants is very good today. Our 10‑year kidney‑graft survival rate, at 75%, is slightly better than the international statistics. In most cases, grafts that lose their function in the long term experience what is known as “chronic rejection,” which represents less of an immunological defense reaction and more a combination of chronic changes in kidney tissue and blood vessels. These eventually lead to impaired renal blood flow and scarring of kidney tissue, thereby reducing filtration capacity. However, patients can live for several years with such impairment without returning to dialysis. In the event of graft loss, a second or third kidney transplantation is pursued. This is technically feasible provided that the clinical requirements, based on the preliminary examinations mentioned above, are met for a new transplant. However, an increased level of immune readiness against the new kidney is to be expected in such cases.
Liver transplantation
The need for liver transplantation arises in many cases of advanced liver disease after all non‑surgical treatment options have been exhausted. In principle, liver transplantation is an option for patients with liver parenchymal diseases, diseases of the bile ducts, certain metabolic disorders, selected liver tumors, acute liver failure, and other, less common causes.
The decision to place a patient on the waiting list for a liver transplantation is made jointly with the referring hepatologist and our colleagues at the University Clinic for Internal Medicine I (Head: Univ. Prof. Dr. Herbert Tilg). In every case, the individual situation of the patient is discussed jointly, and the decision regarding transplantation is made by a multidisciplinary committee. The timing of placement on the waiting list depends on liver function, the progression of the underlying disease, and the general condition of the patient. Criteria considered in the decision‑making process include liver function, the presence of ascites, peritonitis, impaired consciousness, physical weakness, reduced performance or increasing fatigue, bleeding in the stomach or esophagus, the type, size, and recurrence of a tumor, as well as kidney dysfunction caused by the liver disease.
It is important not to place a patient on the transplant waiting list too late, since the outcomes after transplantation also depend on the general condition of the patient before the procedure.
The so‑called MELD score (Model for End‑Stage Liver Disease) is used to assess the severity of liver disease for liver transplantation. The MELD score indicates the severity of the liver disorder.
List of liver diseases for which liver transplantation may be considered:
• Liver parenchymal diseases
Cirrhosis caused by hepatitis B or C, cirrhosis caused by autoimmune hepatitis, fatty liver cirrhosis, cirrhosis of unknown origin
• Cholestatic liver diseases
Primary biliary cirrhosis (PBC), secondary biliary cirrhosis, primary sclerosing cholangitis (PSC), secondary sclerosing cholangitis (SSC), extrahepatic biliary atresia, progressive familial intrahepatic cholestasis (Byler disease), Alagille syndrome, congenital fibrosis, graft‑versus‑host disease (GvHD), chronic rejection, cholestatic sarcoidosis, drug‑induced toxic cholestasis, Caroli syndrome
• Primary metabolic disorders
Alpha‑1 antitrypsin deficiency, Wilson’s disease, hemochromatosis, tyrosinemia, galactosemia, glycogen storage diseases, lysosomal storage diseases, Crigler–Najjar type I, primary hyperoxaluria type I, erythropoietic protoporphyria, primary bleeding disorders (possibly with Budd–Chiari syndrome), urea cycle disorders (e.g., citrullinemia), familial amyloidosis
• Secondary metabolic disorders
in short bowel syndrome
• Acute liver failure
Fulminant viral hepatitis (hepatitis A, B, C, D, E), intoxications, Amanita phalloides (death cap mushroom), paracetamol (acetaminophen), halothane, carbon tetrachloride, ecstasy and others, acute fatty liver of pregnancy, HELLP syndrome, Budd–Chiari syndrome, primary non‑function of a transplanted liver
• Tumors
Hepatocellular carcinoma, neuroendocrine tumors (strict indication required), hepatoblastoma, cholangiocellular carcinoma (strict indication required), liver metastases of colorectal carcinoma (strict indication required)
• Other causes
multiple liver cysts, consequences of severe liver trauma
History
The first liver transplantation in humans was performed in 1963 by T.E. Starzl in Denver, USA. Since then, there have been a number of improvements in diagnostics, surgical techniques, organ preservation, immunosuppression, and the treatment of post‑transplant complications. At the Medical University of Innsbruck, liver transplantation was established by emeritus Professor Dr. h.c. Raimund Margreiter. Under his leadership, more than 1,000 liver transplants were performed, and complex transplantation techniques such as partial liver transplantation, living‑donor liver transplantation, and pediatric liver transplantation were introduced. Today, liver transplantation is the therapy of choice for a wide range of advanced liver diseases. The majority of liver transplants are performed using deceased‑donor organs after removal of the diseased liver. Alternatively, a liver transplant can be performed as part of a “living donation.” In this procedure, part of the liver of a healthy donor is transplanted into the sick recipient. This approach has proven particularly effective in young children (see also pediatric liver transplantation).
Organ allocation
The allocation of organs in transplantation is fundamentally based on two pillars: equity and utility. All patients listed for a liver transplantation have the same right to receive a transplant; urgency and the match between donor and recipient must be taken into account in the selection process.
To meet these two fundamental values, the distribution of organs in many countries, including the USA, Germany, France, Italy, and Switzerland, is based on the Model for End-Stage Liver Disease (MELD) scoring system. Serum creatinine and total bilirubin levels, as well as the INR (International Normalized Ratio), are taken into account to assess the severity of liver disease. In the case of hepatocellular carcinoma, the MELD score reflects the disease-related risk only to a limited extent. For this reason, an “exceptional MELD” of 22 points can be applied at the time of listing if the tumor is within a defined size range (Milan criteria)
The allocation of organs at the Medical University of Innsbruck is primarily based on the MELD score and the severity of disease expressed by it. However, additional factors not represented in the MELD system are also taken into account in the decision-making process, as they likewise play an important role in the outcome:
The waiting time is burdensome for the patient and indicates how long the patient has already been suffering from such a severe disease. In particular, for patients with very similar MELD scores, waiting time is taken into account in the decision-making process.
The following factors are also taken into account in the decision‑making process:
- The compatibility of size, age, and weight
- Suitability of the organ for a “complex” transplantation, for example in the presence of a vascular occlusion (e.g. portal vein thrombosis) or in the case of a retransplantation
- Suitability of the organ for a pediatric recipient
- Distance to the center when the time window is short
- Rate of disease progression
- Liver transplantation as part of a simultaneous transplantation of multiple organs
The allocation of organs is documented and reviewed annually by a delegation of the Ministry of Health together with representatives of the Austrian transplant centers.
Surgical technique
Liver transplantation is now an established procedure performed according to a well‑standardized technique. After opening the abdominal cavity, the liver is exposed. The vessels and bile ducts are then identified; the hepatic artery and bile duct are transected, while the portal vein is initially left intact. The inferior vena cava is then encircled above and below the liver, and the liver is fully mobilized. After clamping and transecting the inferior vena cava and the portal vein, the liver is removed. The new liver is then placed into the abdominal cavity, and the recipient’s vessels are connected to those of the donor organ (inferior vena cava, portal vein, hepatic artery). Once the vascular anastomoses are completed, blood flow is restored and the liver begins to function. Finally, the bile duct is reconstructed by connecting it to the recipient’s bile duct or to a loop of small intestine. Drains are inserted to remove postoperative fluid, and the abdomen is closed. The average duration of a liver transplantation is 4 – 6 hours. After surgery, patients are transferred to the intensive care unit for monitoring and usually move to the transplant surgical ward after a few days, where they are cared for by specially trained nursing staff. The hospital stay after liver transplantation is 2 – 3 weeks.
Liver transplantation in children
Immunosuppression
The immune system recognizes a transplanted organ as foreign. To prevent rejection of the transplanted organ, pharmacological suppression of the immune system is required. A number of medications are available for this purpose. It is crucial to choose the right combination and dosage of drugs. If the recipient’s immune system is insufficiently suppressed, rejection will occur; however, if the dose of immunosuppressive agents is too high, there is a significant risk of infections and malignancies. As a rule, immunosuppression is carried out with three substances. A combination of 1) calcineurin inhibitors (tacrolimus or cyclosporine A), 2) an inhibitor of cell proliferation (mycophenolic acid, mTOR inhibitors, azathioprine), and 3) corticosteroids has proven effective. These medications are part of long‑term maintenance therapy, but their dosage can be significantly reduced over time, allowing good quality of life and physical performance under this treatment. In some cases, an antibody is administered at the time of transplantation or shortly thereafter to reduce the number of immune cells in the recipient and facilitate the initiation of immunosuppression. In the months and years following transplantation, adjustments of immunosuppression are repeatedly necessary. Long‑term follow‑up and medication adjustments are carried out by your hepatologist. It is essential that any significant changes in treatment are discussed with us or with our colleagues at the Department of Internal Medicine I (Head: Univ. Prof. Dr. Herbert Tilg).
Side effects of immunosuppression
Unfortunately, the use of medications to prevent rejection is often associated with the occurrence of side effects. Typical side effects include impaired kidney function, headaches, tremors, diarrhea, elevated blood lipids, gastric ulcers, and osteoporosis. However, unwanted side effects can usually be eliminated or at least reduced by lowering the dose or switching to another medication. Due to the artificially suppressed immune response, there is an increased risk of infection, particularly in the early phase after transplantation. During the first two months after the transplant, additional infection risks should be avoided: large crowds, direct contact with infectious individuals, and heavy dust or dirt exposure should be avoided during this period. In the following months, this risk decreases as the doses of the medications mentioned are reduced.
Results
Since 1977, more than 1,500 liver transplantations have been performed at the Innsbruck Transplant Center. The 1‑year patient survival rate after liver transplantation at our center is 90 – 95%, and the 5‑year survival rate is 70 – 80 %. More than 50 transplantations were performed as living‑donor liver transplants in adults or children. Over 120 transplantations in children have been carried out at our center. In cases of severe transplant rejection that cannot be treated with medication, or in the presence of severe and otherwise uncontrollable vascular or biliary complications, a repeat liver transplantation may become necessary.
Pancreas transplantation
In 1966, the first pancreas transplantation was performed in the United States. Since then, this procedure has evolved from an experimental approach into a standardized and well‑established therapeutic option for patients suffering from insulin‑dependent diabetes mellitus. Continuous progress and improvements in surgical techniques, organ preservation, immunosuppression, and the prevention and treatment of infectious complications have led to the combined kidney‑pancreas transplantation becoming the therapy of choice for dialysis‑dependent, insulin‑dependent diabetic patients today. The goal of pancreas transplantation is to replace the missing insulin production with the newly transplanted organ and thereby achieve a blood glucose level within the normal range through continuous, nearly physiological insulin secretion. In addition to the significant improvement in quality of life associated with successful transplantation, long‑term results show an extension of average life expectancy due to reduced progression or even regression of associated comorbidities.
At the Department of Visceral, Transplant and Thoracic Surgery, more than 590 pancreas transplantations were performed between 1979 and 2016, making the Innsbruck Transplant Center one of the most experienced in Europe in this field.
Indication
According to current knowledge, combined kidney–pancreas transplantation represents the therapy of choice for insulin‑dependent patients with end‑stage renal failure. In addition to the significant improvement in quality of life associated with successful transplantation, stabilization or even regression of diabetes‑related complications (vascular disease, neuropathies) is observed. Furthermore, combined transplantation of both organs prevents the recurrence of diabetic kidney disease. The advantage of combined kidney–pancreas transplantation is confirmed by long‑term outcomes. The average life expectancy of an insulin‑ and dialysis‑dependent patient without transplantation is 8 years. Kidney transplantation increases this to 13 years, while successful combined kidney–pancreas transplantation raises average life expectancy to 23 years. Regardless of the type of diabetes mellitus, only patients who require daily insulin injections and are dialysis‑dependent or have kidney function so severely impaired that dialysis is imminent are listed. The upper age limit is set at 55–60 years, and because overweight increases the risk associated with transplantation, a body mass index (BMI, ratio of body weight to height) below 35 is required.
Pancreas‑only transplantations are also performed. These are indicated in insulin‑dependent patients who do not perceive low blood glucose levels (hypoglycemia unawareness) or who exhibit markedly fluctuating blood glucose values despite regular insulin administration (brittle diabetes). Both conditions pose life‑threatening situations.
Further indications for pancreas‑only transplantation include patients who have already received a kidney‑only transplant or patients with loss of function of a previous pancreas graft.
Surgical technique
After removal, the organ should be implanted into the recipient as early as possible to keep organ damage to a minimum. Typically, the entire organ is implanted together with a portion of the duodenum. The implantation is performed through a midline abdominal incision. The pancreatic graft is connected to an artery in the right lower abdomen and to the inferior vena cava or a mesenteric vein. The duodenum is connected to the recipient’s small intestine approximately 60 cm below the gastric outlet. This technique allows later graft evaluation and biopsy via an upper gastrointestinal endoscopy. In combined kidney-pancreas transplantation, the kidney is connected to the left iliac vessels (artery and vein). The ureter is sutured into the bladder. If the pancreatic graft loses function, retransplantation is also possible. Early pancreatic graft loss occurs in 5 % – 10 % of cases, with the most common causes being vascular thrombosis, intra-abdominal infections, and graft pancreatitis.
Immunosuppression (suppression of the immune response) in pancreas transplantation usually consists of triple maintenance therapy (corticosteroids, calcineurin inhibitors, mycophenolic acid), combined with initial induction therapy using antibodies directed against T-lymphocytes. As treatment progresses, the corticosteroid dose is gradually reduced, with the goal of discontinuing it after 6 to 12 months. The remaining immunosuppressive medications are also reduced and “tailored” by the treating physician so that, over the years, rejection is prevented on the one hand and undesirable side effects are minimized on the other. Impaired kidney function, headaches, tremors, diarrhea, elevated blood lipids, gastric ulcers, and osteoporosis are among the most common side effects. Thanks to an increasingly broad range of immunosuppressive agents, it is usually possible to eliminate or reduce adverse effects through dose reduction or switching to another medication. Due to the strong immunogenicity of the pancreatic graft, dosages are higher than, for example, in liver recipients. Furthermore, fluctuations in drug absorption through the intestine and the varying “propensity for rejection” among recipients require individualized therapies, which are optimized during regular follow-up in consultation with the treating physician. The longstanding close and effective cooperation with the referring internists enables patients to carry out their follow‑up examinations at their local hospital. After receiving the laboratory results and examination findings, any necessary treatment adjustments are made in consultation with our transplant center. Of course, it is also possible to have follow‑up care performed at our center. If rejection is suspected, its extent can be assessed by an upper gastrointestinal endoscopy, during which a tissue sample of the transplanted duodenum is taken and examined. Based on the findings, further treatment is determined. Alternatively, a biopsy of the transplanted kidney may be taken, as rejection usually affects both organs.
Results
The International Pancreas Transplantation Registry (IPTR) collects worldwide outcome data of pancreas transplant recipients. Current figures show a 3‑year patient survival of over 90 %. Depending on the type of transplantation, 3‑year pancreas graft survival ranges between 60 % (pancreas transplant alone) and 80 % (combined kidney–pancreas transplantation). With more than 95% 3‑year patient survival and nearly 90 % 3‑year pancreas graft survival in combined kidney–pancreas transplantation, the results at our clinic are above the international average. Good long‑term outcomes can also be achieved with pancreas retransplantations. The 3‑year graft and 3‑year patient survival rates are 60 % and 85 %, respectively.
Small intestine and multivisceral transplantation
Intestinal transplantation, meaning the replacement of the patient’s own small intestine with a donor small intestine from a deceased or living donor, is the only causal therapy for short bowel syndrome. It represents an alternative to parenteral nutrition and becomes the treatment of choice when complications of parenteral nutrition occur (severe liver dysfunction, catheter‑related infections, loss of venous access). If parenteral nutrition has already caused irreversible liver damage, a combined small intestine–liver transplantation is performed. Multivisceral transplantation refers to the replacement of several abdominal organs (liver, stomach, duodenum, small intestine, colon, pancreas, kidneys). The first human small intestine transplantation was performed in 1967 (University of Minneapolis). In 1989, the world’s first successful multivisceral transplantation in an adult was performed at our clinic by Prof. Raimund Margreiter.
Indication
Small intestine transplantation is the only curative treatment for patients with short bowel syndrome who require lifelong intravenous artificial nutrition. Various diseases can lead to short bowel syndrome. Functional disorders of the small intestine also represent indications; in these cases, impaired intestinal function necessitates artificial nutrition. A combined small intestine–liver transplantation is performed in patients who already have liver cirrhosis. Multivisceral transplantation is a separate indication for patients with Gardner syndrome and for patients with a severely scarred abdominal cavity following multiple previous surgeries.
Occlusion of the vessels supplying the intestine, volvulus, injury/trauma, Crohn’s disease, necrotizing enterocolitis, small‑intestinal atresia, gastroschisis, pseudo-obstruction, malabsorption syndrome, myogenic or neurogenic functional disorders
Surgical technique
After opening the abdominal cavity, all adhesions are first released. In cases of functional disorders of the intestine, it is removed. Suitable blood vessels are then identified and exposed for creation of the vascular connections required for transplantation. The arterial anastomosis is usually established with the abdominal aorta, while the venous connection is made either to the inferior vena cava or to the portal vein/superior mesenteric vein. After restoring blood flow to the new organ, the proximal end of the transplanted small intestine is connected to the patient’s remaining native small intestine, and a connection to the patient’s large intestine is created if it is still present. The distal end of the small intestine is brought out through the abdominal wall as a stoma. Finally, the abdominal wall is closed; in some cases, a temporary abdominal wall reconstruction is necessary. The stoma can be reversed several weeks after transplantation once the small intestine has been accepted.
Multivisceral transplantation
After opening the abdominal cavity, all organs that are to be replaced are first removed. A suitable site on the abdominal aorta is then identified and prepared for the arterial anastomosis. The two major arterial vessels (the celiac trunk and the superior mesenteric artery) are anastomosed together with a segment of donor aorta. Venous outflow of the organ cluster is achieved by replacing the inferior vena cava. After perfusion of the organ block has been established, the connection between the recipient’s stomach and the donor stomach is created. Finally, the intestinal end is connected to the colon and is either temporarily or permanently diverted as a stoma. Closure of the abdominal wall is usually performed in several stages. The stoma can be reversed several weeks after transplantation.
Immunosuppression
To prevent rejection of the transplanted intestine/organ cluster, pharmacological suppression of the immune system is required. The small intestine carries a particularly high risk of rejection; therefore, immunosuppression is administered at higher levels compared with other organs. In addition to the combination of a calcineurin inhibitor (tacrolimus), an inhibitor of cell proliferation (mycophenolic acid), and corticosteroids, an antibody directed against immunoreactive T‑cells is routinely administered intravenously during the first days after transplantation. It is often necessary to intensify immunosuppression in the first weeks following transplantation using high doses of corticosteroids and sometimes repeated applications of anti‑T‑cell antibodies. Immunosuppression is a long‑term therapy; however, the dosage of immunosuppressive agents can be significantly reduced over time, allowing for good quality of life and physical performance under this treatment.
Side effects of immunosuppression
Impaired kidney function, headaches, tremors, diarrhea, elevated blood lipids, gastric ulcers, and osteoporosis are among the most common side effects. Thanks to an increasingly broad range of immunosuppressive agents, it is usually possible to eliminate or reduce unwanted side effects by lowering the dose or switching to another medication.
Because the immune system is artificially suppressed, there is an increased risk of infections, particularly during the initial phase after transplantation. Patients are informed and counseled about this risk during pre‑transplant discussions and throughout their hospital stay. Discharge after transplantation is planned once the patient can feel sufficiently safe in terms of infection risk. During the first two months after transplantation, cautious behavior regarding potential sources of infection is recommended: large crowds, direct contact with infectious individuals, and heavy exposure to dust or dirt should be avoided. In the following months, this risk gradually decreases as the medication doses are reduced.
In addition to the heightened risk of infection, there is also a slightly increased risk of developing malignancies under immunosuppression. In intestinal and multivisceral transplantation, a so‑called graft‑versus‑host reaction may also occur: in this condition, immune cells from the donor organ—which are inevitably transplanted along with the graft—attack the recipient’s organs. This primarily affects the hematopoietic system, mucous membranes, stomach, intestine, liver, and skin.
Results
Worldwide, more than 1,200 intestinal/multivisceral transplantations have been performed. Improvements in immunosuppression and in the prevention and treatment of infections have significantly improved long‑term survival of intestinal transplant recipients over the past ten years. One year after transplantation, the majority of patients are no longer dependent on supplemental intravenous artificial nutrition. One‑year survival is 80 -90 %, and five‑year survival is 60 – 80 %.
Hand transplantation
With the first successful hand transplantation in September 1998, a new era in both reconstructive surgery and transplant surgery began: new, potent medications made it possible for the first time to successfully transplant a hand, a larynx, or an abdominal wall.
Since the first hand transplantation of the “new era” in 1998 in Lyon, France, 33 further transplantations have been performed worldwide in 25 patients. The one‑year survival rate of 100% confirmed not only the feasibility of this complex procedure but also excellent early graft survival of the transplanted hands. It soon became clear that the major obstacles to long‑term therapeutic success were not the surgical procedure itself (a hand transplantation is generally considered technically less demanding than a replantation), but rather immunological factors, medication‑related side effects, and the demanding, lengthy rehabilitation.
The establishment of the International Registry on Hand and Composite Tissue Transplantation (www.handregistry.com) in May 2002 made it possible to analyze the outcomes of all hand transplantations carried out to date. In total, 11 unilateral and 4 bilateral hand transplantations were performed. All recipients were male, with an average age of 32 years at the time of transplantation.
Under the immunosuppressive therapy used, 26 acute rejection episodes (AR) were observed within the first year after transplantation. Despite substantial immunosuppression, AR is therefore a common event after hand transplantation. It is characterized by an exanthema, which may appear irregularly in isolated areas or involve a large portion of the hand. In most cases, rejection could be successfully treated with high‑dose corticosteroid therapy; however, in cases of recurrent rejection, the use of mono‑ or polyclonal antibodies against lymphocytes was required.
Two cases of necrosis of small skin areas, one arterial thrombosis, and in one patient the occurrence of an arteriovenous fistula were observed as surgical complications. All of these complications could be corrected through minor surgical interventions. It has been shown that the required immunosuppression (IS) is roughly comparable to that used after pancreas or heart transplantation. Disadvantages of IS include an increased risk of infections and tumors. In addition to fungal infections of the skin, cytomegalovirus infections were observed particularly frequently.
Analysis of hand function in the transplanted patients demonstrated that almost all patients achieved good functional outcomes. Forearm muscle function enabled grasping ability in all transplanted hands. Grip strength was over 10 kg in 4 patients, more than 5 kg in 8 patients, more than 2.5 kg in 3 patients, and less than 2.5 kg in 2 patients. Finger strength exceeded 2 kg in 4 patients, more than 1 kg in 7 patients, more than 0.5 kg in 2 patients, and was below 0.5 kg in 2 patients.
All patients developed protective sensation, and most additionally achieved good superficial sensibility (Highet scale modified according to Dellon). Overall, sensibility was rated as excellent in 5 hands, good in 2, and satisfactory in 10 hands. In 4 hands, no superficial sensibility was detectable.
However, it remains unclear whether the good functional outcomes are threatened in the long term by chronic rejection. Evaluations of functional, histological, and imaging analyses have so far shown no signs of chronic rejection, yet the risk of such changes occurring over time cannot be definitively ruled out.
General requirements
A hand transplantation is a long‑term and complex therapeutic option. In addition to the surgical procedure, intensive physical therapy and the regular intake of medication to prevent rejection are necessary. After an extensive evaluation process, the waiting time for surgery may be up to two years. The exact timing depends on the availability of a suitable donor and therefore cannot be predicted.
The phase of intensive postoperative care extends over one year, and improvement in functional outcomes continues – based on current experience – for up to five years after the operation. In addition to general suitability for anesthesia, a stable overall health condition is therefore required.
Psychological requirements
A hand transplantation places enormous demands on a patient’s psychological resilience. From the first day – and especially during the first year after surgery – the patient must follow the chosen path with full commitment. Even before the operation, regular exercises to strengthen the forearm muscles must be performed. After transplantation, the recovery of mobility and tactile sensation in the hands can only be expected after months of intensive work.
Furthermore, even with growing experience in the field of hand transplantation, the exact degree of functional recovery cannot be precisely predicted. Based on current knowledge, however, it appears that functional improvement is achievable in all cases compared with the best outcomes obtained with mechanical prostheses.
As with any transplantation, long‑term success depends 100 % on patient cooperation, particularly on daily training and strict adherence to medical instructions, and above all on taking immunosuppressive medication exactly as prescribed.
Surgical requirements
To be able to expect acceptable function after hand transplantation, the amputation stump must be in good condition. This primarily includes the length of the forearm stump, which should in principle be as long as possible, but in any case should amount to one quarter to one third of the original forearm length. The condition of the forearm muscles and the supplying nerves is particularly important, as these structures must be reconnected during the transplantation. From a neurological standpoint, the integrity of the nerves up to the amputation stump is essential.
Logistics and financial feasibility
Although in the past relevant financial support was provided each time by the Medical University of Innsbruck as well as TILAK, a commitment from the patient’s insurance is required due to the multi‑year nature of the undertaking. This applies in particular to approval of the costs for several years of follow‑up treatment and for lifelong immunosuppressive therapy (according to a regimen to be determined exclusively by the Medical University of Innsbruck). Regarding living arrangements, an acceptable—though likely to be determined on a case‑by‑case basis—proximity to the Medical University of Innsbruck should be ensured.
Patient selection
A hand transplantation is a possible therapy for a patient (see prerequisites for hand transplantation) with amputation of the hand and parts of the forearm. Another prerequisite is the intactness of the forearm stump. Primarily, at the Medical University of Innsbruck, only bilateral forearm amputation is considered an established indication for transplantation. However, patients with unilateral amputation are also gladly informed about hand transplantation, and in individual cases these patients may also undergo transplantation. In any case, the patient should have attempted treatment with a myoelectric prosthesis. A hand transplantation is particularly an option for patients who are dissatisfied with such a prosthesis and continue to suffer from the loss of the hand/hands. For the selection of candidates who may be eligible for hand transplantation, specific criteria have been defined in Innsbruck: the first and most important criterion is the patient’s unequivocal desire for therapy. After comprehensive information not only about the operation but especially about the physiotherapy and occupational therapy required during rehabilitation, as well as the need for lifelong immunosuppressive therapy and its associated risks, candidates are given sufficient time to make a final decision. Anatomical prerequisites included the loss of both hands or forearms, and age limits of 18 and 55 years. Candidates had to be in good physical and psychological condition and free of metabolic diseases, infections, and tumors. Examination of the amputation stumps was performed using X‑ray, ultrasound, computed tomography, magnetic resonance imaging, angiography, and CT angiography. To exclude malignancy and infectious foci, examinations of the gastrointestinal tract, the urogenital tract, the teeth, the pharynx, and the paranasal sinuses were carried out in addition to corresponding blood analyses.
The physical therapy was designed to support rapid rehabilitation, reduce swelling and pain, and prevent joint stiffness. “Early Protective Motion – EPM” is a therapeutic concept that had already been used successfully after hand replantation, fulfills the above-mentioned criteria, and was therefore also applied after hand transplantation. Particular attention was also given to regaining sensation and reintegrating the hand into the central nervous system. For this purpose, cognitive exercises according to Perfetti became a central component of rehabilitation. Rehabilitation was supplemented with occupational‑therapy exercises aimed at mastering activities of daily living. Passive exercises were started on the third day after transplantation. Beginning in the third week, the intrinsic hand muscles were activated by electrical stimulation, and one week later active finger exercises were initiated. From the ninth week onward, therapy was expanded to include electromyographic feedback training. Various shaped splints were used to protect the hands and stabilize the wrist. A similar protocol was applied after forearm transplantation. However, since this procedure differed substantially from the first intervention, the rehabilitation program was adapted according to the clinical situation.
Results
The first hand transplantation in Innsbruck was performed in March 2000, five years and six months after the amputation of the hands. Reconstruction was carried out in the following sequence: bones, arteries, veins, flexor tendons, extensor tendons, nerves, and finally skin. The surgical procedure for the second patient was performed in February 2003. Since the amputation had occurred at the level of the upper forearm, the small amount of remaining forearm musculature could not be used for reconstruction. Therefore, the donor’s forearm muscles were attached to the recipient’s upper arm bone. It was the first transplantation of this kind worldwide. The third transplantation was performed in May 2006. The procedure proceeded without major complications, similar to the two previous operations. All surgical procedures were carried out jointly by members of the Department of Plastic and Reconstructive Surgery together with the hand surgery team of the University Clinic for Trauma Surgery. Patient satisfaction. In addition to objective criteria such as motor function or sensation, subjective aspects – such as the ability to interact socially or a holistic sense of bodily integrity after hand transplantation – play a significant role in patient satisfaction. The measurability and comparability of such activities, sensations, and impressions are limited. Therefore, the patient’s own assessment of whether and how the hand transplantation has changed their life is crucial. Furthermore, each evaluation represents a snapshot in time, meaning the timing of data collection is important. As mentioned above, motor function after hand transplantation is clearly superior to that of a myoelectric prosthesis, especially with regard to combined movements and fine motor control, which patients find easier to perform. Of particular importance for both patients is the restoration of bodily integrity, something that the prostheses had not been able to provide.The ability for social interaction as well as for intimate relationships has improved significantly. Overall, both patients are very satisfied with both the functional and cosmetic outcomes. Although motor function after hand transplantation is better than after forearm transplantation in the described patients, it continues to improve, and the observation period for the second patient is considerably shorter than for the first. Overall, the function achieved after hand transplantation amounts to more than 60% of that of a normal hand. Together with very good sensation without cold intolerance, the level of function achieved enables the patient to perform numerous activities that he was unable to manage with prostheses. Thus, he can not only carry out more complex everyday movements, such as buttoning shirts or picking up small coins from a flat surface, but his self‑confidence and thus his interaction with his surroundings have also improved significantly. So far, no signs of functional deterioration or the onset of chronic rejection have been observed. The patient describes his daily life as completely normal; he is fully reintegrated and engaged in his profession. With the transplanted hands, he has already undertaken several transcontinental motorcycle trips. The goal was therefore achieved, and from the patient’s perspective, the decision to undergo hand transplantation was clearly the right one.
The outcome after forearm transplantation is also satisfactory, although several episodes of acute rejection and medication side effects complicated rehabilitation. In the meantime, a stable situation has been achieved, and all side effects have been resolved. The result is impaired by insufficient sensation. However, it remains to be hoped that further functional improvement will occur in this regard.